Silicon nitride (Si3N4) is a typical strong covalent bond compound, which not only has high melting point, high hardness, wear resistance, but also high flexural strength and good thermal conductivity. It has an irreplaceable position in key fields such as national defense, military industry, and electronic information.
The preparation of silicon nitride ceramics first requires silicon nitride powder with good performance, and has the following characteristics: 1) The finer the particle size of the micropowder, the higher the specific surface area, which is more conducive to the progress of sintering, thereby forming a more uniform microscopic Therefore, the particle size of the silicon nitride micropowder should be small, and the average particle size should be at least submicron; 2) If the crystal form of the silicon nitride micropowder is equiaxed, the density of the green body will be greatly improved, so , the powder must contain more granular α-Si3N4, so that there is enough α-phase to be transformed into β-phase during sintering, so that the ceramic body can obtain good physical properties; 3) The purity of the raw material powder must be high and cannot contain Too many impurities will greatly reduce the mechanical properties of silicon nitride products.
Preparation method of silicon nitride ceramic micropowder
The preparation method of silicon nitride ceramic micropowder mainly includes direct nitridation method of silicon powder, carbothermic reduction of silica method, chemical vapor phase synthesis method, and thermal decomposition method.
Silicon powder direct nitridation method
Silicon powder direct nitridation method, that is, Si powder reacts with N2 to form Si3N4 powder. The chemical reaction is:
3Si(s)+2N2(g) → Si3N4(s)
The synthesis of Si3N4 by this reaction is relatively simple, and does not involve side reactions that generate impurity phases. Since this reaction is a strong exothermic reaction (about 822.5kJ of heat can be released for every 1 mol of Si3N4 generated at 1200°C), in the actual reaction process, the temperature of the Si(s) surface is much higher than the temperature set for the reaction, so the reaction interface is The Si will liquefy and vaporize to form Si(l) and Si(g), and at the same time, Si(l) and Si(g) can also be nitrided to form Si3N4. During the whole reaction process, the release of part of Si(g) or the accumulation of Si vacancies will generate pores inside the Si particles, while the Si3N4 shell will be formed on the surface of the Si particles, and sintering and agglomeration will occur between the Si particles, as shown in Figure 1. shown.
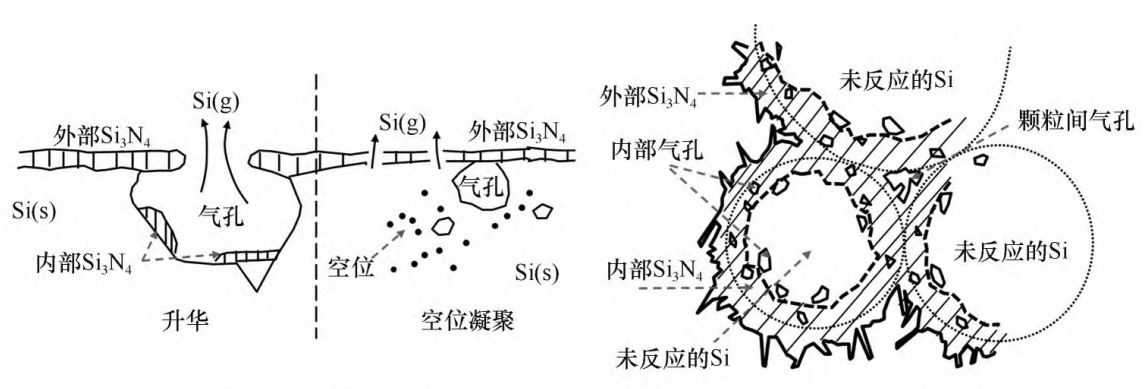
Fig. 1 Schematic diagram of the nitridation reaction process
According to the strong exothermic characteristics of the direct nitriding method of silicon powder, the self-propagating combustion technology is mainly used to prepare Si3N4 powder in the industry. Then, the nitridation reaction is initiated by ignition and the subsequent nitridation reaction is induced by the heat released, as shown in Figure 2(a), (b). The most significant advantages of this technology are energy saving and economy. Only the initial ignition heat is provided, and the subsequent reactions proceed spontaneously. In theory, high-quality Si3N4 powder can be prepared by using ultra-fine, electronic-grade high-purity Si powder. However, the apparent activation energy Ea during the combustion process is 292~670kJ/mol, which indicates that it is still subject to diffusion and mass transfer, and the temperature gradient during the self-propagating combustion process is large (2400~25℃), resulting in part of the Si powder being liquefied by nitrogen Part of the Si powder is wrapped in the liquid phase and cannot be nitrided, some of the Si powder is only nitrided on the surface, some of the Si powder has cracks on the surface and is nitrided, and some of the Si powder is vaporized and then nitrided, so as to obtain free Si. The bulk Si3N4 is shown in Figure 2(c), (d) and a small amount of Si3N4 fine powder is shown in Figure 2(f).
![]()
Fig. 2 Schematic diagram of the synthesis of silicon nitride by self-propagating combustion reaction and product morphology
Carbothermic reduction of silica
The raw materials of this method are quartz powder of certain purity and high-purity carbon powder (coke or charcoal). The carbon undergoes a reduction reaction to generate elemental silicon and then react with nitrogen or ammonia to obtain silicon nitride. The reaction formula is as follows:
SiO2+C+N2 → Si3N4+CO
SiO2+C+NH3 → Si3N4+CO+H2O
The advantage of the carbothermic silica reduction method is that the obtained micropowder has a small particle size and high purity, and contains a large amount of α phase, the reaction process is simple, and the speed and efficiency are higher than that of the direct nitridation method. The reaction should be carried out with an excess of carbon, so that there is no residual silica unreacted. When the reaction is over and the product is about 600°C, the excess carbon can be removed by burning. The disadvantage of this preparation method is that it is difficult to completely reduce and nitride the silica, and the residual silica will greatly affect the high temperature performance of the ceramic.
The carbothermic silica reduction method has been successfully commercialized. At present, domestic and foreign companies with 100-ton production lines mainly include Japan’s Toshiba, Japan’s Sumitomo Chemical, Fujian Zhenjing and Hengyang Kaixin. The quality of powder sold by each company is shown in the table. 1 shown. It can be seen from the data in the table that the α phase, C content and metal impurities all meet the requirements of high-quality powder, but the O content in the powder is relatively high. This is mainly because the bonding force of Si and O is stronger than that of Si and N, and it is difficult for the Si-O bond in the SiO2 powder to be completely replaced by the Si-N bond, resulting in part of O remaining in the lattice. Therefore, it is generally difficult to obtain a powder having an O content of less than 0.9% by mass. This type of powder can be used to prepare structural ceramics or mold release agents in the photovoltaic field that do not require high thermal conductivity. It is difficult to prepare high thermal conductivity ceramics because oxygen impurities in the lattice will scatter phonons and reduce thermal conductivity.
Application Research of Silicon Nitride Ceramics
The application research of silicon nitride ceramics is carried out with the continuous breakthrough of sintering technology, and the performance of ceramics is also becoming more and more excellent. At present, silicon nitride ceramics mainly include dense ceramics and porous ceramics. With the increasing demand for new materials with excellent high temperature performance in modern industry, the development speed of silicon nitride ceramics has increased significantly, and the application has become more and more extensive.
Dense Silicon Nitride Ceramics
Substrate material
The theoretical thermal conductivity of silicon nitride ceramics can be as high as 200-320W/m K, and silicon nitride has high strength, high hardness, high resistivity, good thermal shock resistance, low dielectric loss and low expansion coefficient, etc. It is an ideal heat dissipation and packaging material.
Bearing material
Rolling fatigue life is an important indicator to measure the performance of bearing materials. Among the common structural ceramics, the rolling fatigue life of silicon nitride is significantly higher than that of zirconia, silicon carbide, alumina and other materials, and it is also the most suitable for bearing materials. Silicon nitride precision ceramic bearings have been used more and more in precision transmission systems such as electroplating equipment, high-speed machine tools, medical equipment, chemical equipment, low temperature engineering, and wind power generation.
Abrasives
Silicon nitride has high hardness, Hv=18-21GPa, HRA=91-93, second only to a few superhard materials such as diamond and cubic boron nitride. The coefficient of friction is small (<0.1), and it is self-lubricating, similar to the metal surface of oil. In the ultrafine powder and food processing industries, the performance of silicon nitride ceramic grinding media balls is higher than that of traditional grinding balls, with higher hardness and superior wear resistance. Because of its very low consumption, the grinding cost and the degree of powder pollution are reduced.
Metallurgical materials
Silicon nitride ceramics have excellent oxidation resistance, and the oxidation resistance temperature can be as high as 1400 ℃. It is stable in a dry oxidizing atmosphere below 1400 °C, and the operating temperature can generally reach 1300 °C. In neutral or reducing atmosphere, it can even be successfully applied to 1800℃. In humid air at 200°C or dry air at 800°C, silicon nitride reacts with oxygen to form a surface protective film of silicon dioxide, which hinders the continued oxidation of silicon nitride. And silicon nitride material can be used in the working conditions of rapid cooling and rapid heating, and has a wide range of applications and huge development space in the metallurgical industry.
Mechanical engineering
Traditional metal materials, involving valves, cutting tools, cylinder liners, grinding media, wear-resistant bushings, bearings, various nozzles, etc., are not resistant to high temperature, easy to wear, and easy to rust. Replaced by new ceramic materials. The silicon nitride ceramic material has excellent wear resistance, corrosion resistance, and high temperature thermal shock resistance, which can be competent in this field.
Porous Silicon Nitride Ceramics
Filter material
Filter materials have certain requirements for filtration characteristics, mechanical properties and chemical stability. Porous ceramic materials are not only widely used in gas purification and filtration, but also can effectively filter various types of solutions. Porous silicon nitride ceramics have the characteristics of adjustable pores, good corrosion resistance and chemical stability, which are good foundations for their use as filter materials.
Wave-transmitting material
Wave-transmitting material is a multi-functional dielectric material that can not only reduce the loss of radio frequency electromagnetic waves, but also can well resist harmful environmental influences such as external rain and snow. It can be used in radar radomes and antenna windows. The basic requirements are: good wave penetration, high stability, little influence on radar signals, and good mechanical properties and corrosion resistance. For such applications, porous silicon nitride ceramic materials have shown great potential. By adjusting the dosage and pore size of the pore-forming agent, it can be used as a sandwich material for broadband radomes. The aerospace field is also one of the applications of wave-transmitting materials. The use of porous silicon nitride ceramic materials can improve the performance of radar, so it is also crucial for the improvement of military equipment.
Bone replacement material
Bioceramic materials need to have strong physical properties such as compression resistance and wear resistance, and good bio-histocompatibility when implanted in living organisms is also a key factor to note. Porous silicon nitride ceramic material has a porosity similar to that of human bone tissue, and has no cytotoxicity, which meets the biological requirements of orthopaedics and can be used as an excellent bone substitute material.
Catalyst carrier
The catalyst carrier is usually the skeleton of the active component of the catalyst, which plays the role of support and support. It generally does not have catalytic activity itself, and sometimes acts as a catalyst. Because of its many types, it has good applications in different fields. The catalyst carrier is required to have certain adsorption and plasticity, as well as certain thermal stability and mechanical strength. As a porous ceramic material, porous silicon nitride ceramics have the characteristics of high strength and good chemical stability, which meet the requirements of catalyst carriers.
Epilogue
In recent years, with the continuous development of China’s manufacturing and high-end mechanization, the demand for silicon nitride materials is increasing day by day. Ultrafine powder has become a research hotspot in the field of inorganic non-metallic materials.
As far as the preparation of silicon nitride micropowder is concerned, the future development direction can be started from the following four aspects:
(1) Raw materials: Better raw materials should be found, or the physical and chemical properties of raw materials should be improved; greener, environmentally friendly and low-cost silicon and nitrogen sources should be selected to control the particle size and uniformity of raw materials.
(2) Additives: Appropriate diluents and additives should be selected to assist the nitridation reaction to improve the performance of silicon nitride micropowder.
(3) Purity: Impurity content should be strictly controlled to improve the purity of silicon nitride micropowder; at the same time, appropriate amount of beneficial impurities that are conducive to sintering should be excavated.
(4) Reaction conditions: The conditions for nitriding reaction should be improved, such as nitriding temperature, nitrogen pressure and flow rate, reaction atmosphere and reaction equipment, etc., to obtain ideal silicon nitride micropowder.
