1. Introduction of fumed silica:
Fumed silica is one of the most important high-tech ultra-fine inorganic new materials. It is porous, non-toxic, tasteless and pollution-free. The average primary particle size is about 7-40 nanometers, the specific surface area is 50-380 m2/g, and the product purity is high. , SiO2 content is not less than 99.8%, is a multifunctional additive.
Silicon dioxide (SiO2) is very abundant in nature. Sand, crystal, quartz sand, silica powder, diatomaceous earth, etc., the main components are SiO2; in synthetic silica, precipitated silica, fumed silica, etc., the main component is also SiO2. Like sand, microsilica and diatomaceous earth, these are natural products, some of which are applied directly without processing, and some are processed into the market, but the application fields of the products are very limited; precipitated silica ( Silica) and fumed silica (silica) are the most widely used chemically prepared silica products. Although the amount of precipitation silica is relatively large, the application field of fumed silica is wider. . In the market, the price of fumed silica products is also the highest (except as jewelry and collectible crystals), and the application fields involved are also the most extensive.
2. Fumed silica production process:
Fumed silica is an ultra-fine powder material obtained by the high-temperature hydrolysis and condensation of halosilanes in a oxyhydrogen flame (Fig. 1 is a schematic diagram of the synthesis principle of fumed silica). Due to its unique preparation process, it has a different structure and unique properties from other silica products.
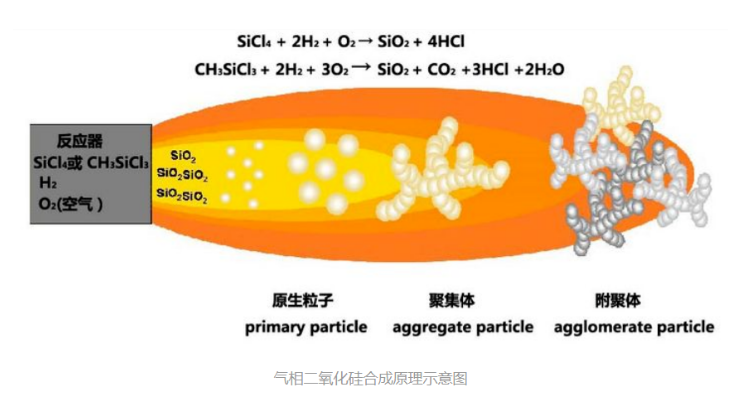
The production process of fumed silica has the following characteristics:
(1) In terms of raw materials, the raw materials for synthesizing fumed silica are very simple, one is halosilane (SiCl4 and CH3SiCl3) and combustible gas (hydrogen and oxygen (air)), which effectively prevents other substances from being brought into the product.
(2) In terms of technology, the halosilane is vaporized first and then enters the reactor. The temperature of the hydrolysis reaction is also around 2000 °C, and then the processes of aggregation, separation, and deacidification are completed at high temperature. The whole process is in a continuous, sealed The system is completed to ensure that the product is free from external influence and pollution.
(3) All key links of the entire production unit are made of high temperature, acid and alkali corrosion resistant materials, which can prevent other pollution sources from entering the product during the production process.
The characteristics of the raw materials and production processes for the production of fumed silica make the fumed silica products have small particle size (primary particle size 7-40nm), large specific surface area (100-400m2/g), and high surface activity (surface Contains highly active Si-OH groups); the fumed silica products prepared under this special process also have a special three-dimensional dendritic structure, and the product has high porosity. In appearance, fumed silica appears as a “smoke”-like fluffy white ultrafine powder, so it has the English name of fumed silica.
3. The structure and properties of fumed silica:
So what are the unique structures and properties of fumed silica?
(1) Unique “three-dimensional dendritic” structure
In the production process of fumed silica, halosilane is first hydrolyzed and condensed into individual silica particles, and then gradually grow into spherical particles of 7-40 nanometers, which are called “primary particles” of silica. ).
The “primary particles” continue to move forward with the direction of the flame in the reaction furnace, and the particles collide with each other. At this time, because the temperature in the reaction furnace is still relatively high, the particles are still close to the molten state. Particles with a three-dimensional dendritic structure in which multiple spherical particles are fused together are called “Aggregate Particles” of silica. Since the particles in the aggregates are fused to each other, they are stable structures that are almost impossible to separate.
Silica “aggregates” continue to move forward with the airflow in the pipeline, collide, and then connect with each other to form flocculent fluffy powders called “agglomerates” of silica (Agglomerate Particles) . Due to the low temperature in the pipeline at this time, the connection between the “aggregates” is only connected by physical adsorption, which is an unstable structure, which can be separated (dispersible) under a certain mechanical force. .
(2) High surface activity
During the high-temperature hydrolysis and condensation process of fumed silica, some silanols (Si-OH) remain on the surface, and the presence of silanols on the surface makes the surface of fumed silica stronger and more active.
In addition, the existence of silanol groups also provides the possibility of surface modification of fumed silica. Surface modifiers with different structures are selected to react with silanol groups, and some functional groups are grafted to the surface of fumed silica, thereby making The functions of fumed silica are more diverse.
(3) Small particle size and large specific surface area
The “primary particle size” of fumed silica is 7-40nm. What is the concept? We can understand from the data comparison in the following table. If we enlarge a fumed silica particle to the size of a standard football, we need to enlarge it by about 30 million times; if we enlarge the football by about 30 million times in the same proportion, it will become about the size of Mars.
The particle size of fumed silica is small, which makes its specific surface area very large. Usually, the specific surface of fumed silica is 100-400m2/g. Usually our house of 150m2 has an indoor area of about 120m2. Even if we use fumed silica products with the lowest specific surface area currently on the market (such as HL-150), less than 1g, its specific surface area can cover the entire interior of the house. area! And if it is like a product with a high specific surface area (such as HL-380), with only 18.8g of product, its specific surface area is equivalent to the area of a standard football field! It is precisely because of the characteristics of small particle size and large specific surface area of fumed silica that it has good adsorption performance and can be widely used in catalysts, food, medicine, thermal insulation materials and other fields. and other functions.
4. The application of fumed silica:
Fumed silica is amorphous, with high purity (SiO2% ≥ 99.8%), the presence of surface active silanols, and the low bulk density and good fluidity of fumed silica. Surface modification by dry method is possible, and surface modification can be achieved without destroying the special structure of fumed silica. By selecting surface modifiers with different structures and functions to modify it, different functional products can be obtained. Realize the functionalization of fumed silica.
This series of unique structures and properties of fumed silica makes it show excellent reinforcement, thickening, thixotropy, anti-settling, anti-sag, adsorption, heat insulation and other functions in practical applications. , coating, adhesives, industrial catalysis, food, medicine, CMP, thermal insulation materials and other dozens of fields have a wide range of applications, making fumed silica truly become the “white and rich beauty” in the silica and the market’s “white” darling”.
